___________________________________________________________________________
PAPER I
THE SHAPE OF THE VERTEBRATE EMBRYO CAN BE MIMICKED BY PUNCTURING AN OVOIDAL BALLOON
_____________________________________________________________________
THE SHAPE OF THE VERTEBRATE EMBRYO CAN BE MIMICKED BY PUNCTURING AN OVOIDAL BALLOON
Stuart Pivar and Peter Sheesley
ABSTRACT
The shape of the vertebrate embryo can be mimicked by the recoil pattern of a tense elastic ovoidal membrane. The vertebrate blastula is a tense, elastic ovoidal bilayer membrane. It is demonstrable that a puncture at the ventral midline posterior of this figure will expand anteriorly and dorsally, generating a border outline curve that is congruent with the spiral configuration of the vertebrate embryo. This Euclidian demonstration of geometrical similarity by congruence of model and life supports the candidacy of the model as the origin of biological form.
ARGUMENT
The problem of morphogenesis remains the principal theoretical concern in biology. It is not known to science how the embryo is formed. The problem occupies thousands of scientists at any time. The focus of theoretical research has changed with the currency of ideas relative to evolution and genetics. The focus du jour is mechanobiology which attributes the origin of embryonic form to mechanical forces on the dividing cells, in full rejection of a genetic code. Still, no one has made head nor tail of the patterns generated before our eyes by the dividing egg cells.
The present demonstration is based on the supposition that gastrulation, the landmark event in embryogenesis, consists of the consequences of the bursting and elastic recoil at the ventral midline of the tense blastula membrane. As the border expands the concurrent anterior-dorsal trajectory of recoil generates a predictable typical curve that resembles the profile of the general vertebrate embryo.
The demonstration is based as well on the predictable hydrodynamic behavior of the separate, interconnected segments of the embryonic structure that predict the migration of the fluid content from high to low pressure containments, reminiscent of the famous physics demonstration with two interconnected balloons. The embryonic balloon experiment generates the spiral form of the embryo.
REFERENCES
ILLUSTRATIONS
_____________________________________________________________________
PAPER II
THE ORIGIN OF THE VERTEBRATE MUSCULOSKELETAL BODY PLAN IN THE MECHANICAL DEFORMATION OF THE PATTERNED MEMBRANE OF THE BLASTULA
The Origin of the Vertebrate Musculoskeletal Body plan in the mechanical deformation of the patterned membrane of the blastula
Stuart Pivar, Mark McMenamin, David Edelman, Peter Sheesley
ABSTRACT
This paper demonstrates that the serial subdivision of a spherical cell through the three axes of space generates a geometrically patterned spherical membrane, which upon rupture is catastrophically deformed into a figure congruent with that of the vertebrate embryo.
We propose that the form of the vertebrate musculoskeletal system is the result of the topological deformation of the self-organized geometrical pattern of separate banded girdles resulting from the serial binary fission of the egg cell, which constitutes the membrane that encloses the blastosphere, and that the embryo is formed by the sudden deformation of the patterned membrane by the catastrophic rupture of the ventral midline.
The algorithm is presented by mechanical drawings. These show that the simple topological deformation of a spherical membrane consisting of seven separate girdles produces a figure that is congruent with the archetypal vertebrate body form, by step-by-step comparison with the classically observed steps of avian embryology.
HISTORY
That the origin of the appendicular skeleton is in the pectoral and pelvic girdles that encompass the spherical blastocoel has been known to embryology since the nineteenth century. Due to insurmountable difficulties in the observation of transparent embryonic cells, little is known about the structure of the first ball of cells, called the blastula, nor that of the inner cell mass from which the embryo appears like a developing image in a photographic plate. Although the genes have been identified that initiate the process of limb development, the formative mechanism guiding the process has remained unknown.
The proposed algorithm accounts for the formation of the blastula and for the deformation of the blastula in the event that forms the embryo.
The term gastrulation, meaning stomach formation, is in practice applied to the observed event common to animalia where the initial ball of cells enters its own interior to form a bilayer sphere. While the space of the interior of the ball was historically taken for the stomach primordium, this space is in fact resorbed quickly and has no place in morphogenesis. Hence the term gastrulation a misnomer in this application.
PREMISE
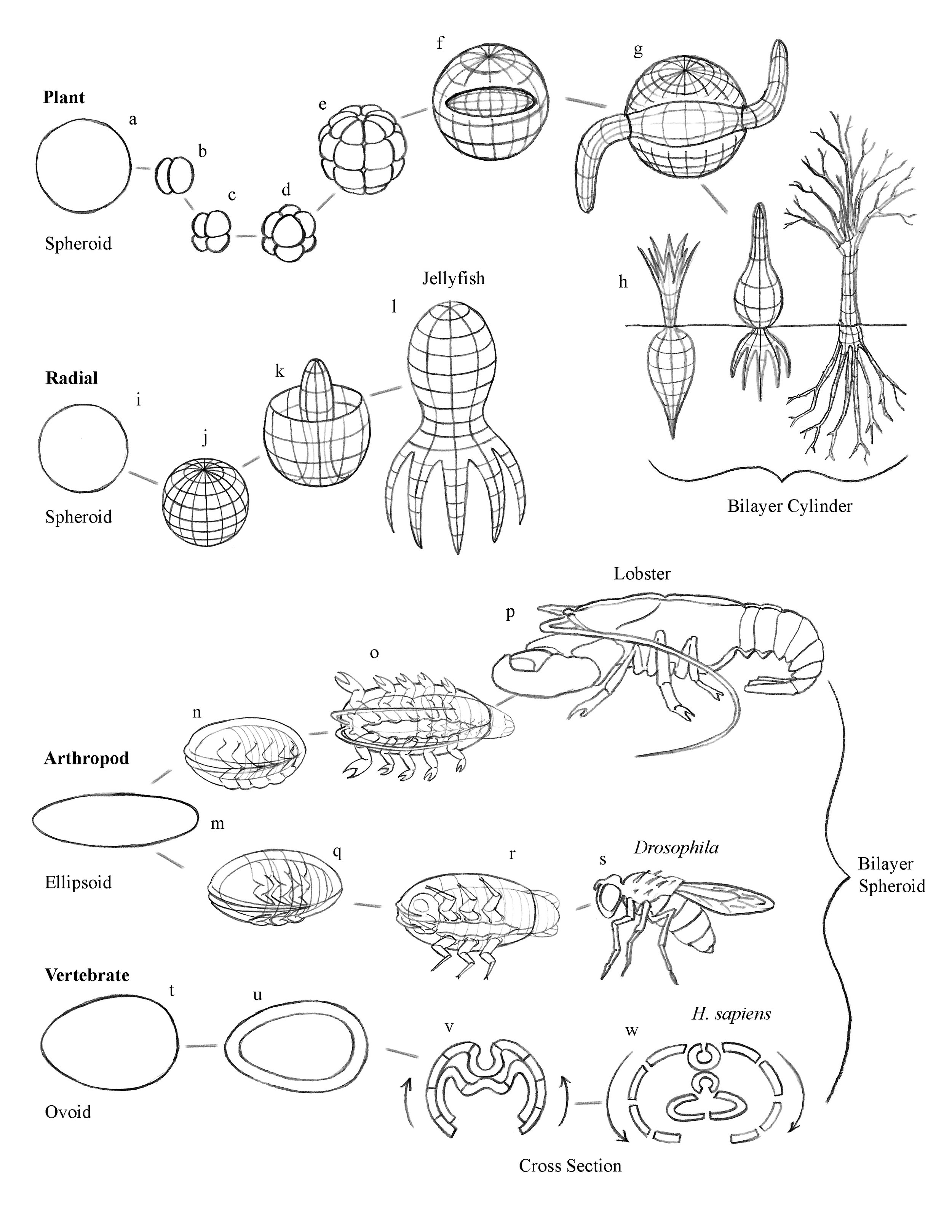
We demonstrate that the serial subdivision of a spherical cell through the three axes of space generates a geometrically- patterned spherical membrane, which upon rupture is catastrophically deformed into a figure congruent with that of the vertebrate embryo (Fig. 1 — for simplicity the gastrulation step is not depicted in the animation of the subject algorithm–it occurs as step “e” ).
The blastula membrane is comprised of an equatorial girdle, two polar caps, and two girdles in the space between the poles and the equator, coincidentally analogous to the pattern of the terrestrial climate zones. The girdles of the lower hemisphere are the familiar pectoral and pelvic girdles. The polar cap is the sacrum. The upper hemisphere forms the skull, comprising occipital, parietal, and frontal girdles, and the nasal polar cap.
The girdles may slide freely over the surface of the embryonic blastular sphere, analogous to terrestrial continental drift. The embryo is formed as the tense blastula membrane shrinks toward the dorsal midline upon the catastrophic rupture of the blastula pattern along the ventral midline, whereupon the following transformations occur:
The pectoral and pelvic girdles form the limb;
The occipital girdle forms the cranium, the jaw and the incisors;
The parietal girdle forms the maxilla and the canines;
The frontal girdle forms the front of the face, the zygomatic, and the molars;
The nasal cap forms the nose.
DEMONSTRATION
The blastula assumes its spherical shape from the serial binary fission of the egg cell, with ten subdivisions producing approximately a thousand cells. These thousand cells then self-organize into seven circumferential bands, or girdles.
While the pectoral and pelvic girdles are recognized in embryology as the origin of the limbs, in this thesis three additional segments of the embryo are recognized as girdles–the occipital, parietal, frontal and nasal. Heretofore these have not been recognized as such in embryology.
The rupture of the ventral midline of the tense blastular membrane cause ventral-dorsal and anterior-posterior recoil of the membrane. The latter force causes the three anterior girdles to bend and fold toward the anterior, one within the other, like Russian dolls, from posterior to anterior, creating three telescoping concentric pairs of jaws, bursting through the anterior wall of the nasal girdle. The latter folds outward forming a cuff, notable in dogs (Fig. 2). Concurrently, the posterior hemisphere reveals the rib cage, as the two appendicular girdles shrivel toward the dorsal and anterior as arms, legs, and vertebrae.
The prognathic front of the posterior girdle passes through the two forward girdles causing them to telescope over each other, forming the three skull bands that bare the three ranks of teeth. The two tense forward girdles separate at the ventral midline and retreat dorsally, creating the zygomatic arch (Fig. 3).
The three prognathic fronts pull apart top from bottom, forming teeth: the occipital forms the incisors; the parietal forms the canines; and the frontal forms the molars, with each deriving from different skull bones.
The embryological event called gastrulation is known to shape the embryo. Here the steps of gastrulation are described by the algorithm of the premise:
The tense blastula membrane parts along the ventral midline, causing the membrane to shrink and retreat toward the dorsal and anterior sides;
The pectoral and pelvic girdles form the appendicular skeleton;
The posterior polar cap is displaced toward the anterior, ending up sandwiching the spine at the pelvic girdle;
The posterior edge of the occipital girdle is displaced to the anterior by passing through the inside of the anterior edge, turning the girdle inside-out;
The occipital girdle membrane passes through the parietal and frontal girdles causing them to displace toward the anterior as the dorsal side of the membrane retreats to the posterior, completing the inversion;
The resulting figure is observed to be a topologically congruent replica of the archetypal vertebrate body form.
CONCLUSION
This paper reports the discovery of a simple topological algorithm whereby an elastic spherical membrane can assume the form of the embryonic vertebrate musculoskeletal system by a single deformation. The success of this algorithm to mimic development is the basis for the nomination of the algorithm as a solution to the question confronting science of the origin of complex living form and the mechanism of evolution.
The relevance to life of this geometrical device is that the blastula–the ball of cells resulting from the subdivision of the egg–is possessed of the characteristics of the sphere posited by the algorithm. The steps of the deformation of the surface are imitated in classically observed textbook avian embryology (Figs. 5-6), providing a sufficiency of evidence for proof of principle. The simplicity of the sequence of events makes detailed textual concordance redundant. As the material is unrelated by definition to the state of the art of developmental science, there is little need for literature references.
Fig. 5
Fig. 6
Fig. 7
Fig. 8
Fig. 9
Fig. 10
PAPER III
THE MECHANOBIOLOGICAL ACCOUNT OF VERTEBRATE LIMB DEVELOPMENT BY THE TENSILE ELASTIC FAILURE OF THE EMBRYONIC LIMB GIRDLES
THE MECHANOBIOLOGICAL ACCOUNT OF VERTEBRATE LIMB DEVELOPMENT BY THE TENSILE ELASTIC FAILURE OF THE EMBRYONIC LIMB GIRDLES
Stuart Pivar, Mark McMenamin, and Peter Sheesley
ABSTRACT
This paper presents the discovery of a mechano-geometrical model accounting for the developmental formation of vertebrate limbs. The theorem demonstrates that limb development is the result of the elastic midline rupture failure of the limb girdles under tension caused by the enlargement of the underlying blastocoel.
The model demonstrates that the result of the application to failure of a force of tension in one direction upon an elastic orthogonal grid is a figure congruent with the archetypal vertebrate limb. The well known embryonic pectoral and pelvic girdles are composed of an elastic grid of cells.
KEY WORDS
Mechanobiology, blastula, gastrulation.
HISTORY
The study of limb development over centuries has amassed data serving the interests of anatomy in its widespread value in science and art. Although drawings of the steps of embryogenic development have existed since the seventeenth century, the accurate depiction of the steps of embryology were not perfected until the end of the nineteenth century by such noted workers as Wilhelm His Sr.
But the mechanism directing the complex ballet of embryonic cells that forms the organs remains a mystery. The embryologist is confronted with microscopic scale micron-thin, optically-indifferent membranes. Steps starting with limb buds are seen to seamlessly transform into hands and feet, much as an image appears on a photographic plate in a developing tray. Embryologists can literally make heads or tails of it, but little more.
In recent years, under the rubric of mechanobiology a sizeable segment of researchers have investigated non-genetic phenomena as candidate mechanisms of development. This paper presents a theory of limb development by non-genetic, mechanical means.
PREMISE
Limb development can be accounted for as the outcome of the deformation of predictable self-organizing geometrical patterns that occur in the stacking of cells produced by serial rounds of cell division.
Embryology describes development of the embryo as the deformation of the initial ball of cells, called the blastula. The structure of the blastula is unknown except for the appearance of two peripheral girdles–the pectoral and the pelvic–that originate the limbs.
Beginning with the premise that the blastula is in the form of a ball of an elastic tissue of rows and columns of cells self-organized in bands, the limbs are shown to be formed as two of the bands pull apart while the ventral midline bursts and separates. The dorsal recoil trajectory of the tense membrane of the two appendicular girdles causes the grid pattern to shrivel, forming the observed limb buds.
The steps of embryogenesis can be understood as though the blastula membrane is a thin elastic sheet stretched over a growing sphere. The parting of the ventral midline precipitates the elastic recoil of the two limb girdles, in resemblance of the deflating of a balloon. The predictable steps of this catastrophic snap-back are the reverse of the observed steps of vertebrate limb development.
COMMENTARY
Epigenesis is the sole mechanism guiding all-natural events, excepting those guided by human intelligence, which it can be argued is ultimately epigenetic as well.
The first three divisions of the fertilized egg cell are plainly visible to the microscope eye. The resulting eight cells of a theoretical cuboid are squashed together in their confining enveloping cortical sphere, in the embryological event called compression. Subsequent divisions create an optically incomprehensible drama that forms the ball of cells, called the blastula. Limb development begins when the pectoral and pelvic girdles each pull apart and separate at the ventral midline.
The first few divisions of the egg create simple forms that clearly occur by the mechanical forces on the cells, without any ostensible need for a guide. While the epigenetic stacking of cells can account for simple shapes, it is hard to conceive that an entire organism can be formed by the repeatable stacking of millions of cells. The claim of mechanobiology to generate the complex organism without the guidance of a genetic code is made plausible by the discovery of intercellular phenomena that can serve as clues, such as cell adhesion.
The gradual appearance of the image of a hand in the tissue of the embryological hand plate is like seeing an image appear on an exposed photo print sheet in the developing tray. To know the genes that trigger limb development is as useless as knowing the chemicals in the developing solution when it comes to understanding both kinds of development.
The presentation of the model is by a series of mechanical drawings depicting a hypothetical model of the unknown embryonic stages of limb development that are shown to accurately predict the musculoskeletal structure of the limb. The power of the algorithm to accurately predict nature surpasses coincidence.
The format of the presentation of the model is that of the Euclidean theorem where a hypothesis is proposed that can account for a phenomenon for which the cause is unknown. The phenomena of development are topological and geometrical. Data is graphic. This model is a developmental fate map which has no quantitative measurements and hence no quantitative data.
DEMONSTRATION
The mechanical account of limb development records the steps of the shriveling of the gridded pattern that composes the membrane of the pectoral and pelvic girdles. The topological deformations include:
The formation of the long bones by the axial rolling of a flat band of tissue, followed by the formation of split cuffs by axial compression;
The accordion failure of the limb axis by four or more bends;
The formation of claws by the 360 degree rotating trajectory of the digit tips that displace the symmetrical pattern of concentric circles at the tip to the locus of the paw-pads, fingerprints, and the extraneous prominences on limbs of tetrapods;
The appendicular musculoskeletal system in analogue with the accordion failure of a bilayer cylinder;
The accounting for the polymorphism in the vertebrate limb in the neotenic retardation or acceleration versus growth in ontogeny and phylogeny.
CONCLUSION
Limb development has for long offered a convenient laboratory model organism for developmental science. The presumed goal is to discover a system that can produce the form of the limb from axiomatic causes–the blueprint for the limb. This paper is the publication of the claim of primacy in the discovery. A corollary is that a code for the body in the genes is redundant.
REFERENCES
As the material presented is by definition unrelated to the state of the art of developmental science there is no continuum of theory.
Gould, S.J. Ontogeny and Phylogeny. Belknap Press of Harvard University Press, 2003.
Lewontin, R.C., It Ain’t Necessarily So: The Dream of the Human Genome and Other Illusions, New York Review of Books, New York, 2000.
Fields, C., and M. Levin. 2020. Does Evolution Have a Target Morphology? Organisms: Journal of Biological Sciences, vol. 4, no. 1, pp.57-76. DOI: 10.13133/2532-5876/16961.
Levin, M., and V. Bush. 2020. Reading and writing the morphogenetic code. Allen Discovery Center at Tufts University, Foundational White Paper.
Bessonov, N., M. Levin, N. Morozova, N. Reinberg, A. Tosenberger, V. Volpert. 2015. On a Model of Pattern Regeneration Based on Cell Memory. PLoS ONE 10(2): e0118091. https://doi.org/10.1371/journal.pone.0118091
Giaimo, C., 2020. When These Sea Anemones Eat, It Goes Straight to Their Arms. The New York Times. www.nytimes.com/2020/09/05/science/sea-anemones-arms.html.
. 2020. MRI scans of the brains of 130 mammals, including humans, indicate equal connectivity. Tel Aviv University. https://medicalxpress.com/news/2020-07-mri-scans-brains- mammals-humans.html
Serra, M., S. Streichan, M. Chuai, C. J. Weijer, L. Mahadevan. 2020. Dynamic morphoskeletons in development. Proceedings of the National Academy of Sciences. 117 (21) 11444-11449; DOI: 10.1073/pnas.1908803117
Sereno, M.I., et al. 2020. The human cerebellum has almost 80% of the surface area of the neocortex. PNAS. doi.org/10.1073/pnas.2002896117.
Wagner, D.S., K.R.W. Matthews. 2019. Human Embryo Research: What do we know and how do we know it? Rice University’s Baker Institute for Public Policy. https://www.bakerinstitute.org/media/files/files/fcd4841d/chb-pub-greenwall-her-022519.pdf
Bessonov, N., M. Levin, N. Morozova, N. Reinberg, A. Tosenberger, V. Volpert. 2015. On a Model of Pattern Regeneration Based on Cell Memory. PLoS ONE 10(2): e0118091. https://doi.org/10.1371/journal.pone.0118091
Burrows, L. 2020. Researchers use geometry and dynamics to better understand tissue organization. phys.org. https://phys.org/news/2020-05-geometry-dynamics-tissue.html
ILLUSTRATIONS
The copious illustrations have been accumulated over many years. The relative simplicity of the models makes detailed textual concordance redundant.
Figure 1
The overall limb developmental trajectory.
a, the pectoral and pelvic girdles in the blastula membrane
d, separation of the ventral midline
f, bone formation
g, axial accordion fold
Figure 2
a-m, limb development pattern
n-s, cross-section of neural tube formation
Figure 3
Bone formation
Figures 4, 5
1-11, the concurrent limb development and inversion as the inner cell mass
Figure 6
Schematic drawing of limb development
Figure 7
The origin of the musculoskeletal limb joint system in the compression accordion fold of the limb axis
Figure 8
Limb bone formation by axial warping and compression
Figure 9
Scapula and pelvis formation
Figure 10
Resume of vertebrate development
Figure 11
Formation of primate-form digits
Figure 12
Limb variants
Figure 13
The recoil phenomenon
Figure 14
The origin of fingerprints
Figure 15
Schematic analysis of digit development
Figure 16
Limb development polymorphism
Figure 17
Resume of limb development
PAPER IV
The Formation of the Neural Tube by the elastic recoil of the blastula membrane during gastrulation
The Formation of the Neural Tube by the elastic recoil of the blastula membrane during gastrulation
Stuart Pivar, Mark McMenamin, and Peter Sheesley
ABSTRACT
This paper presents a solution to the persisting problem of how the neural tube is formed. The claim is made that the neural tube is formed as the mechanical consequence of the elastic recoil of the tense, elastic blastula membrane upon bursting along the ventral midline, caused by the expansion of the blastocoel. The proof of principle is demonstrated by the comparison of drawings of the steps of the proposed hypothetical mechanism for vertebrate embryogenesis with observed classic avian embryogenesis.
DISCUSSION
The history of the investigation of the origin of the neural network, beginning with the discovery of the neural crest by Wilhelm His Sr. in 1868, has led to the recent discoveries that the formation of the neural tube, the somites, and other early embryonic structures are triggered by certain identified genes. But the mechanisms by which the organs are shaped has remained unexplained.
The appended mechanical drawings are presented as “blueprints” for the development of the neural tube as well as the notochord and the gut tube, all shown as the consequence of the catastrophic bursting and the elastic recoil of the blastula membrane that forces the linear invagination along the dorsal midline, in response to lateral tension. The subsequent rejoining of the separated margins of the ventral midline form the notochord and gut tube by comparable mechanical reactions to lateral stress.
This algorithm provides a mechanical account for the embryonic process of gastrulation, (gr., stomach formation). The fundamental tubes of the body are demonstrated to be formed by the deformation of the blastula in gastrulation.
DEMONSTRATION
The proof or principle of the proposed solution to the problem of neurogenesis is by the demonstration of the congruence of the steps of the hypothetical algorithm with the steps of observed avian embryogenesis. The demonstration consists of the comparison of the classical cross-sectional images of the trunk of the vertebrate embryo with the comparable sequence in the hypothetical model.
The events depicted in the hypothetical model are an epigenetic sequence where each stage is the mechanical result of the previous stage, comparable to any event in nature not guided by an inherited plan or a superior intelligence. Here development is seen as the consequence of the bursting of the balloon-like blastula and the predictable ways the bands of cells comprising the blastula membrane are deformed during the catastrophe.
Neurogenesis begins with the bursting along the ventral midline of the blastula membrane followed by the recoil toward the dorsal. Lateral compression forces the membrane to invaginate along the dorsal midline, sealing the margins to form a tube enclosing a cable of membrane fibers (Figs.7-9), and gathering in square bales called somites, the shriveled, compressed remains of the pectoral and pelvic girdles, and other organs. These will regain their pre-gastrulation form in the succeeding steps of limb development.
For the elemental simplicity of this solution to the development problem we forego detailed textual explanation of the plates.
CONCLUSION
This theorem including a graphic account of organogenesis has no precedent in the literature. Attempts are made to explain the origin of the neural tube consisting of the identification of the genes that initiate development, and to map the migration of neural crest cells as they form the neural network. This line of investigation has remained merely descriptive.
The vertebrate embryo first appears in the inner cell mass, the image forming as from nowhere like a photographic image in the development tray. To complete the analogy; to know the chemicals of photographic development does not explain how the image is formed. To know which genes initiate development does not explain development.
PAPER V
EARLY EMBRYOLOGY PRESERVED ON THE TURTLE SHELL
EARLY EMBRYOLOGY PRESERVED ON THE TURTLE SHELL
Stuart Pivar and Peter Sheesley
ABSTRACT
This paper establishes that the turtle’s shell is an enlarged replica of its own blastula, confirmed by the discovery of a topological algorithm that predicts the patterns of the turtle shell from a blastula simulacrum model, demonstrating that the development process, quantified by sequential cell division, results geometrically in the formation of a multilayer sphere that generates new layers internally, as the outer layers are either jettisoned or accumulate as pyramidal stacks.
The turtle’s shell is the preserved form of its blastula. So is that of the armadillo, sturgeon, snail, clam, and the dorsal plates of crocodile, and most dinosaurs. These taxa preserve the gridded ball of cells comprising the outer layer of the embryonic bilayer blastula, as the inner layer–termed the inner cell mass–forms the rest of the body.
The development of the blastula may be read from the pyramidal strata comprising the shell of the turtle, each scute a stack of preserved stages of embryogenesis, the apex of each representing an early stage of cell division. The pattern of the carapace of the turtle may be reproduced topologically by the compression of a hexagonally paved sphere. The annular rings of trees are an analogous preserved record of the quantified stages of plant embryology. The succulent plant Discoides elephantides grows like a turtle shell.
The development process is quantified by sequential cell division that results geometrically in the formation of a multilayer sphere or cylinder that generates new layers internally, as the outer layers are either jettisoned or accumulate as pyramidal stacks. The boundary membrane of the developing animal body is a multiple layer sphere, adding from the inside and casting off the burst layer outside, exemplified by the locust or crab crawling out of its instar, or retaining the layers as pyramidal strata.
The plant blastula is an elongated spheroid forming coaxial cylinders that develop from the inside out, squeezing meanwhile through the interstices as branches, leaves and flowers.
Fig. 1
Fig. 2
Fig. 3
PAPER VI
The Simulation of the Archetypal Flower and Fruit Form by a Novel Topological Algorithm
The Simulation of the Archetypal Flower and Fruit Form by a Novel Topological Algorithm
Stuart Pivar, Mark McMenamin, and Peter Sheesley
ABSTRACT
The archetypal anatomy of the flower—consisting of the central tubular pistil terminating in the stigma, surrounded by the symmetrical display of pollen-bearing anthers within a circlet of petals and sepals—is the universal given of botany. Yet botany, since its ancient inception, has remained a generally taxonomic and descriptive science because no plausible account for the origin of the unique morphologies of the leaf, flower or fruit has been forthcoming. This paper presents a hypothetical construction originating in an amoeboid primordial ancestor cell that predicts the form of the organs of the flower with sufficient accuracy to qualify as a candidate solution to the problem of the origin of the form of the organs of the archetypal flower.
The paper reports the discovery that the anatomy of the archetypal flower and fruit may be simulated by a simple topological construction consisting of the deformation of the self-organized patterns that occur in an axially elongated toroidal surface, in imitation of the form of the streaming amoeboidal locomotion exhibited by the living amoeboidal cell. These patterns are demonstrated in a series of experiments with models consisting of inflatable toroidal balloons fabricated from thin-gauge, transparent polyvinyl-chloride film. Inflated with a fluid, the models assume a unique terminal topological structure that exhibits motility in unique patterns. An illustrative sequence of schematic mechanical drawings presents a hypothetical topological transformation by the mechanical failure of the surface resulting from the application of compressive and tensile forces acting on its parts. The separated parts are seen to be congruent with the organs of the archetypal flower and fruit. The algorithm provides a novel account for the origin of the structures that comprise the flower and fruit. The experiment suggests that the organismal form of the archetypal flower and fruit may originate in the self-organized patterning of the embryonic membrane generated in the toroidal streaming of the living or ancestral germ plasm, predecessor of the seed.
KEY WORDS: Pseudopodal locomotion, sol-gel interphase, Urform
INTRODUCTION
The May 2016 issue of the journal BioScience presents an extensive article summarizing the state of the art of the understanding of the origin of plant anatomy (Álvarez-Buylla, 2016). The article concludes:
‘Even though genetic approaches have been favored, it is recently being accepted that the richness and robustness of biological forms are not encoded in the genes but rather emerge from their interactions and non genetic components…. A fundamental challenge in systems biology is developing the mathematical and computational approaches to explore how the different levels of complexity are coupled.’
The gorgeous, extravagant forms of flowers and fruits have engendered wonderment since Aristotle’s pupil, Theophrastus, called the “father of botany,” wrote De Causis Plantarum. As to the origin of the geometrically regular shapes and forms of flowers and fruits, attempts at the mathematical rationalization of plant form flourished with the natural idealists of the age of reason following the schools of thought of von Haller, Bonnet, Oken, and epitomized by Johann Wolfgang von Goethe–who saw the living form as a dynamic system built of an interpenetrating formative principle. In The Metamorphosis of Plants, Goethe postulates that all flower parts are derived from leaves (Goethe, 2009). Goethe coined the term “morphology.”
In his letters, Goethe famously proclaimed his discovery of the Urpflanze as the Protean progenitor of all botanical form (Goethe, 1992). Thus, Goethe sought to reproduce in thought what nature accomplishes in the formation of its works. On May 17, 1787, Goethe wrote to Johann Herder:
‘Moreover, I must confide to you that I have come very close to the secret of the generation and organization of plants and that it is the simplest thing one can imagine. The archetypal plant will be the most extraordinary creature in the world, for which nature herself will envy me. With this model and the key to it one will then be able to invent plants ad infinitum that must be consistent. In other words, even if they do not exist, they could exist and are not merely painterly or poetic whims but possess an inner truth and necessity. It will be possible to apply the same law to all living things.’
Goethe’s theory of plant metamorphosis and his approach to natural science inspired the search for the ideal form that directed goals of the 19th century developmental biologists. Exampled are the morphologists who attempted to rationalize the vertebrate skeleton, including the accepted evolutionary phylogenetic account of the visceral arches in fish and the complex head, ear, and neck bones of later vertebrates. Goethe proposed the controversial theory of the origin of the skull in fused vertebrae. He distinguished himself as an anatomist by his discovery of the intermaxillary bone in humans, establishing our species relative to the other primates.
Unfortunately, Goethe neglected to describe the Urform or Urpflanze, much as Fermat neglected to include the proof to his famous “Last Theorem,” or Poincaré his conjecture. Moreover, following elaboration of the theory of evolution called the Modern Synthesis in the early decades of the 20th century, the search for the origination of form came to be sought at the molecular level, while classical morphology was largely neglected (Maresin, 1985; Presnov, 1988). Modern attempts at rationalizing the morphology of the flower bring to bear the contemporary state of the art of the mechanics and mathematics of the expanding tissue of cells during development (Mahadaven, 2013; Coen, 2013).
The mathematics of the spiral patterns of the centers of composite flowers such as sunflowers and daisies has been studied since ancient time, including its relation to the Golden Section and Fibonacci series. This involves the arrangement of multiple blossoms and seeds within the flower and is irrelevant to the morphology of the flower archetype, the subject of the present study.
This paper reports the discovery of a unique self-organizing topological structure that occurs in a streaming elongated toroidal membrane. The deformation of this patterned structure leading to the dehiscense, or bursting failure along predictable failure lines is demonstrated to generate an accurate simulacrum of the archetypal flower and fruit morphology. The formation of patterns is experimentally demonstrated in the surfaces of fabricated models. The patterns are demonstrated as congruent with the corresponding plant parts.
THE MODEL
The form assumed by a membrane-bound colloidal fluid is exemplified by the phenomenon of amoeboid pseudopodal locomotion. While the study of this phenomenon has not provided a clear understanding of its cause, investigators favor the theory of toroidal streaming, as a sol-gel interphase phenomenon (Mast, 1923). The transparency of moving fluid surfaces makes observation vague, resulting in varying interpretations of what is seen by the investigator as to what governs the process. The following is an idealized generalization of the morphology of pseudopodal locomotion by toroidal sol-gel membrane.
The protoplasm mass generates an enveloping membrane. A weakness in the wall results in a circular port from which a newly formed membrane radiates as it emerges from the port. Sol-state material exits immediately to undergo gelation, producing additional membrane that radiates to flow backwards over the body, and to re-enter at a posterior port. Upon re-entry, the membrane wrinkles in a radial pattern-like that formed when a coat sleeve is turned inside out generating a tube within a tube. The premise of this theoretical construction is that the pattern generated upon re-entry of the inner tube produces a radially segmented membrane that envelops the fluid contents from posterior to anterior (Fig. 1, a-d).
The algorithm is demonstrated experimentally by the inflation of fabricated elongated toroidal surfaces. While the inflation of a spherical balloon produces an ever larger sphere, the elongated toroidal surface on inflation achieves a terminal radial configuration as the internal tube segments the interior with radial membranes, like an orange, each surmounted distally by a thin tube (Fig. 2).
ANALYSIS
The achievement of this unusual shape by the inflation of the toroidal balloon is demonstrated in the familiar toy called a water-tube, a 10cm. toroidal balloon of thin polyvinyl-chloride film (Fig. 2, g-i). Filled with water, glycerine, or air the model demonstrates amoeboid locomotion and clearly shows the internal membrane configuration that is achieved on full inflation, notably including thin axial tubes that self-organize in a radial pattern and that are capable of conducting a fluid axially, separate from the fluid-filled interior. The radial cross-section is demonstrated by freezing the water-filled model and sawing it in half transversely.
This demonstration describes the mechanical failure of the model toroidal membrane in terms of the corresponding parts of the flower and fruit:
Seen vertically erect, (Fig. 1, e) the streaming anterior of the pseudopod advances like the growth of the plant shoot (Fig. 1, a-g). The coaxial tubes of the pro-androecium are seen subject to the expansion of the interior surface within an outer cylindrical inelastic constraint (Fig. 1, h). The walls of the androecium are compressed laterally, breaking them into separate columnar structures that split open, exposing the pollen within (Fig. 1, k).
Basal-distal tension causes the androecium to part from the gynoecium at the point where inner segmentation commences. This reveals the surface of the stigma (Fig. 1, l). The tips of the petals withdraw axially and anteriorly, the distal mid-point formed as the filament tube parts and the basal half adheres to the anthers, as the filament. The petals and stamens bend outward to form the finished flower (Fig. 1, k-m).
The universal configuration of the fruit is a bulbous tube, radially segmented, the segments separated in many species by membranes. Seeds come to lie within the segments remaining attached to the central axis. This configuration is shown to be the final stage in the growth of the central canal of a torus that passes through the encompassing tubular canal of an outer torus (Fig. 3, a). The origin of the seed is accounted for by the hypothesis of the radial expansive growth of a secondary toroidal tube within the central axis of the primary toroidal tube (Fig. 4, m, s).
The Geometrical/Topological Development of the Organismal Flowering Plant
The development of flowering plants, i.e., shrubs and trees, may be understood as the axial extension of the toroidal membrane originating in the germ plasm (Figs. 5-8). Indeed, the fundamental vascular structure of a tree trunk is the phloem/xylem configuration of concentric cylinders, the sap rising in the xylem and descending in the phloem. The model presumes coaxial toroidal membranes, one within the other contiguously, the inner membrane producing branching by extrusion through the interstices of the outer membrane (Fig. 5). The near-universal spiral configuration of branch and twig patterns may be accounted for by a twist in the axis of the inner membrane (Fig. 6). The organismal shoot and root configuration of the shrub and tree may be accounted for by the upward and downward growth direction of opposite ends of the originating toroidal germ plasm (Fig. 7). The morphology of the leaf may be understood as the separation of the upper axis of an ellipsoidal extrusion of the inner membrane (Fig. 8).
The recent description of a fossil fruit of the pteridosperm Caytonia sp. from South Hadley Falls, Massachusetts (201.2 million years old, McMenamin 2016) is strongly in accord with the model of telomation as shown here in Fig. 8. The oblique placement of integumental longitudinal fibers in the Caytonia sp. cupule represents a case intermediate between the parallel (lily) and pinnate (ash) models presented here for extrusive growth of thorns and leaves. The telomation model shown in Fig. 8, with the morphogenetic grid specifying sites of extrusive growth (enations), can also, for the first time, provide an adequate explanation for extinct plants with bilateral symmetry such as zosterophylls (McMenamin and McMenamin 1994). The toroidal bauplan in these plants experienced dorsal-ventral flattening, thus imparting a recognizable bilateral symmetry.
DISCUSSION
The argument that the embryonic membrane is toroidal is based on recent work of experimental embryologists (Beloussov, 2006; Presnov, 2006; Kraus, 2006). Jockusch and Dress called the structure a “multi-torus.” The following models of toroidal membrane flow are based on predictions of plausible flow patterns of viscous fluids (Jockusch, 2003).
The slow toroidal streaming of an inelastic tubular membrane generates longitudinal exo-vaginations in the interior surfaces, as the surface is condensed in the incurrent vortex funnel by self-organized folds to accommodate the reduced perimeter maintained by lateral compressive forces, as the outer surface enters the interior (Fig. 2).
Tubular vortices occur naturally in many fluid structures and are predictable by the laws of fluid mechanics, although this presentation presents them descriptively without underlying mathematical corroboration. Toroidal streaming may plausibly generate two, three, four, or five tube vortices distributed radially at the perimeter of the inner vortex (Fig. 2, a-f).
The description of this algorithm is based on the supposition that the tissue of cells acts as an empatterned membrane. The subdivision of a single cell into a mosaic of many cells is considered here to be morphologically irrelevant. The change from cell membrane to cellular tissue is ignored in the sequential illustrations for reasons of clarity. That which is actually a tissue of cells is depicted as acting as a membrane.
CONCLUSIONS
Flowers, by the wondrous combination of symmetry of form, gorgeous colors, and delightful perfumes, capture the attention and enhance the lives of all. Flowers are studied by a myriad of organizations, both horticultural and scientific. Yet, the literature includes no coherent account of the origin of their universal archetypal form. While the theory proposed in this paper may not be considered a rigorous proof, the accuracy of the predictive power of the hypothetical model proposed should offer encouragement to botanists in further investigation.
The riddle of embryogenesis has been sought for thousands of years by observation of the forms of the adult, larva, embryo, egg, and seed. The answer may lie in the predecessor of the egg, the primordial germ plasm, an amoeboid wanderer cell, separate from the soma, common to all life, established classically by Weismann as the basis for cell systematics. The primordial germ plasm may well be the bearer of the Goethean Urform.
The idea that the universal form of the plant blossom is the manifestation of an underlying topological, mathematical law of order, or property of space, only enhances the wonder of this glorious product of nature.
REFERENCES
Álvarez-Buylla, E.R. 2016. Systems biology approaches to development beyond bioinformatics: Nonlinear mechanistic models using plant systems, BioScience, May 3.
Beloussov, L. and Grabovsky, V. 2006. Morphomechanics: goals, basic experiments and models. International Journal of Developmental Biology, 50: 267-275.
Coen, E. 2013. Cells to Civilization: The Principles of Change That Shape Life. Princeton: Princeton University Press.
Goethe, J. W. 2009. The Metamorphosis of Plants. Boston: The MIT Press.
Goethe, J. W. 1992. Italian Journey: 1786-1788. New York: Penguin Classics.
Jockusch, H. and Dress, A. 2003. From sphere to torus: A topological view of the metazoan body plan. Bulletin of Mathematical Biology, 65:57-65.
Kraus, Y.A. 2006. Morphomechanical programming of morphogenesis in Cnidarian embryos. International Journal of Developmental Biology, 50:267-275.
Liang, H. and Mahadevan, L. 2013. Growth, geometry, and mechanics of the blooming lily. Proc Natl Acad Sci USA, 108(14):5516-21.
Maresin, V.M. and Presnov, E.V. 1985. Topological approach to embryogenesis. Journal of Theoretical Biology, 114(3):387-398.
Mast, S. O. 1923. Mechanics of Locomotion in Amoeba. Proc Natl Acad Sci USA, 9(7):258-261.
McMenamin, M. A. S. 2016. Dynamic Paleontology. Springer, Cham, Switzerland.
McMenamin, M. A. S. and D. L. Schulte McMenamin. 1994. Hypersea: Life on Land. Columbia University Press, New York.
Presnov, E.V., Isaeva, V.V. and Chernyshev, A. 2006. Topological patterns in metazoan evolution and development. Bulletin of Molecular Biology, 68:2053-2067.
Presnov, E.V., Malyghin, S.N. and Isaeva, V.V. 1988. Topological and thermodynamic structuress of morphogenesis. Thermodynamics and Pattern Formation in Biology (Lamprecht, I. and Zotin, A.I., Eds.). Berlin: Walter de Gruyter, 337-370.
Ronse De Craene, L. 2010. Floral Diagrams, An Aid to Understanding Flower Morphology and Evolution. Cambridge: Cambridge University Press.
Raghavan, V. 2000. Developmental Biology of Flowering Plants. Springer.
Reid, R, 2007. Biological Emergences, MIT.
FIGURE LEGEND
Fig. 1 Flower morphogenesis from primordial germ plasm to flower and fruit. a, Germ plasm; b-d,
Axial extension; e-g, Roots/shoot differentiation; h-m, Flower/fruit development.
Fig. 2 Schematic drawings and photographs of model toroidal membrane. a-f, Schematic diverse
radial symmetry patterns of primordial germ plasm demonstrating thin axial tubes; g-i,
Photographs of vinyl toroidal membrane models.
Fig. 3 Ovary/fruit formation. a, Initial coaxial toroidal form; b-e, Withering of flower; f, Ovary/ fruit; g-i, Seed development; j, Generational reiteration.
Fig. 4 Schematic cross-section of primordial germ plasm membranes. a-l, Cross-section of various
fruits; m-r, Coaxial configuration of toroidal membranes; s-t, Schematic coaxial configura-
tion of membranes.
Fig. 5 Stem and branch morphology. a, Initial coaxial configuration of toroidal membranes; g-j, Extrusion of twig and branch forms; k, Flower meristem primordium.
Fig. 6 Origin of twig patterning. a-d, Invagination; e-f, Axial twist; g-i, Shoot; j, Twig/leaf pattern.
Fig. 7 Organismal model of the flowering plant. a-g, Shoot/root differentiation at opposite ends of
germ plasm; h-j, Leaf development; k-n, Flower development.
Fig. 8 Leaf morphology. a-d, Coaxial toroidal membranes; e-i, Extrusive growth of thorns and leaves; j-s, Leaf development.